Struggling with fluids that break down under extreme conditions? The cost of equipment failure from poor lubrication is a constant worry, forcing you to compromise between performance and fluid longevity.
Isononanoic acid is a key building block for high-performance synthetic esters, like polyol esters. Its unique branched structure provides exceptional thermal, oxidative, and hydrolytic stability, which are the core chemical principles for optimizing advanced engineered fluids and extending equipment life.

A laboratory setup with beakers and flasks, representing chemical formulation of engineered fluids.
That’s the short answer, but as formulators, we know the real value lies in the details. Understanding why this specific C9 acid outperforms others and how its molecular architecture translates directly into superior performance is what separates a good formulation from a great one. To truly master your craft, you need to go beyond the data sheet and grasp the fundamental chemistry at play. From my experience, a deep dive into these principles can unlock new levels of performance and reliability in your products. Let’s explore the chemical science that makes isononanoic acid an indispensable tool for modern fluid technology.
Why is Isononanoic Acid a Superior Building Block for Polyol Ester Lubricants?
You select a standard ester for your formulation, but it fails under extreme heat and pressure. This compromises performance and leads to premature breakdown, forcing costly and frequent fluid changes.
The solution lies in the molecular architecture. Isononanoic acid’s unique branched structure (3,5,5-trimethylhexanoic acid) provides outstanding thermal stability and prevents crystallization at low temperatures. This makes it a superior building block for robust polyol ester (POE) synthetic lubricants that won’t let you down.

A molecular model of isononanoic acid highlighting its branched structure.
To appreciate why isononanoic acid (INA) is so effective, we first need to look at the basics of polyol esters (POEs). These are high-performance synthetic lubricants created through a chemical reaction called esterification. In this process, a poly-functional alcohol, known as a polyol (like Neopentyl Glycol, Trimethylolpropane, or Pentaerythritol), reacts with one or more carboxylic acids. The choice of acid is what ultimately defines the final properties of the lubricant. While traditional linear fatty acids can be used, they have a major drawback: their straight-chain structure allows the molecules to align and pack together neatly, causing them to crystallize at low temperatures. This leads to the lubricant thickening or even solidifying, which is a critical failure point in cold-start situations or in refrigeration systems. This is where INA’s genius lies. Its highly branched, bulky structure creates what chemists call steric hindrance. Imagine trying to stack a pile of perfectly straight logs versus a pile of crooked tree branches; the branches simply can’t pack together tightly. This physical barrier at the molecular level dramatically improves low-temperature fluidity and lowers the pour point of the final lubricant, ensuring it flows freely when it’s needed most.
How Does INA’s Molecular Structure Dictate Lubricant Performance?
Your lubricant degrades chemically under stress, leading to sludge, varnish, and increased acidity. This isn’t just physical wear; it’s a molecular breakdown that shortens the fluid’s life and damages equipment.
INA’s lack of beta-hydrogens prevents a key thermal degradation pathway, while its branched structure provides steric hindrance. This combination results in superior thermal, oxidative, and hydrolytic stability, directly translating to longer-lasting, more reliable lubricants that resist chemical breakdown.

A diagram illustrating the stable carbon backbone of isononanoic acid with a less stable linear acid.
The exceptional performance of INA-based lubricants isn’t magic; it’s a direct result of its specific molecular design. As formulators, understanding these structural advantages allows us to predict and engineer fluid behavior with precision. Let’s break down the three pillars of stability that INA provides.
Thermal Stability and the Absence of Beta-Hydrogens
One of the most common failure modes for esters at high temperatures is a reaction called beta-elimination. This occurs when the acid portion of the ester has hydrogen atoms on its second carbon (the beta carbon). These hydrogens are a chemical "weak spot" that can be stripped away under heat, causing the ester molecule to break apart. The structure of isononanoic acid, 3,5,5-trimethylhexanoic acid, is specifically designed to prevent this. It has no hydrogens on its beta-carbon, effectively removing this degradation pathway. This inherent structural integrity means INA-based esters can withstand much higher temperatures without coking or forming deposits, a critical feature for applications like jet engines and industrial ovens.
Oxidative Stability
Oxidation is the enemy of lubricant life. It’s a reaction with oxygen that causes the fluid to thicken, form sludge, and become acidic. This process is often accelerated by heat and the presence of metal catalysts. INA’s advantage here is its fully saturated structure. It has no carbon-carbon double bonds, which are highly reactive sites prone to attack by oxygen. By building a lubricant with a saturated building block like INA, you are creating a fluid that is intrinsically more resistant to oxidative breakdown, leading to significantly longer drain intervals and cleaner systems.
Hydrolytic Stability
Hydrolysis is the breakdown of an ester in the presence of water. The bulky, branched structure of INA provides a powerful defense against this. This steric hindrance acts as a physical shield, protecting the vulnerable ester linkage at the core of the molecule from being attacked by water molecules. In applications where water contamination is a risk, such as in some hydraulic or refrigeration systems, this enhanced hydrolytic stability is crucial for preventing fluid degradation and the formation of corrosive acids.
What Makes INA Essential for Extreme Temperature Applications?
In a jet engine or an industrial compressor, the operating conditions are brutal. Extreme temperatures and pressures can cause standard lubricants to fail, leading to catastrophic equipment damage and significant downtime.
Isononanoic acid-based polyol esters are engineered for these extremes. For aviation, INA provides the critical thermal stability to prevent coking at high temperatures. For refrigeration, it offers essential low-temperature fluidity and miscibility with modern HFC/HFO refrigerants.
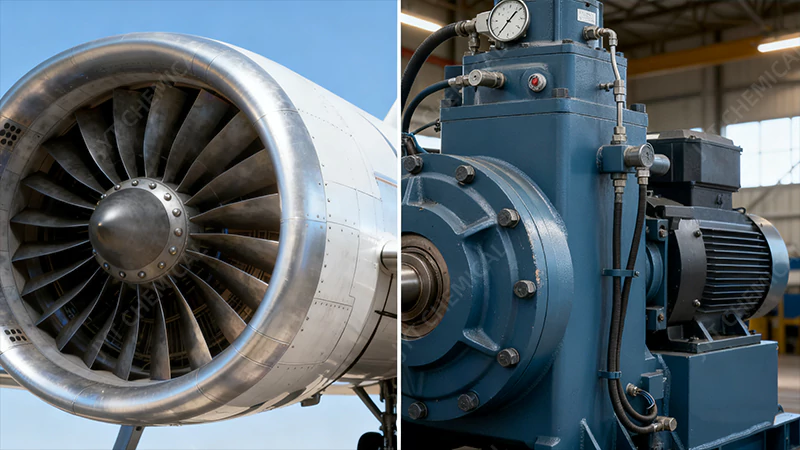
A split view showing a jet engine turbine and a refrigeration compressor unit.
The true test of a lubricant’s chemistry is its performance at the extremes. Isononanoic acid has become the foundation for fluids in two of the most demanding fields: aviation and refrigeration.
High-Performance Aviation Lubricants
Aviation gas turbines operate at bulk oil temperatures that can exceed 200°C, placing immense thermal and oxidative stress on the lubricant. In this environment, lesser synthetic oils would quickly break down, forming hard carbon deposits (coking) that can block oil lines and nozzles, leading to engine failure. POEs synthesized with isononanoic acid are the industry standard for a reason. Their exceptional thermal stability, derived directly from INA’s lack of beta-hydrogens and saturated structure, allows them to resist degradation and maintain engine cleanliness. This ensures that bearings and gears remain lubricated and cooled effectively, extending engine life and maximizing the time between costly overhauls. The result is a safer, more reliable engine that can withstand the rigors of modern flight.
Advanced Refrigeration Lubricants
The refrigeration industry has undergone a massive transformation, shifting towards environmentally friendly refrigerants like HFCs and HFOs. These new refrigerants are more polar than their predecessors and are not compatible with traditional mineral oil lubricants, leading to poor oil return and compressor failure. This is where INA-based POEs shine. They possess the right level of polarity to be miscible (able to mix) with these modern refrigerants across a wide temperature range. This miscibility is crucial for ensuring that oil, which inevitably circulates with the refrigerant, can return to the compressor. Without proper oil return, the compressor would be starved of lubrication and fail. Furthermore, the excellent low-temperature fluidity imparted by INA’s branched structure ensures the lubricant doesn’t solidify in the coldest parts of the system, like the evaporator, maintaining system efficiency and reliability.
Can Isononanoic Acid Also Function as a Powerful Corrosion Inhibitor?
You’re formulating a water-based coolant or metalworking fluid, but the water causes rust on expensive parts and machinery. This leads to component damage, fluid spoilage, and costly production downtime.
Yes, it can. When neutralized to form a salt, isononanoic acid becomes a highly effective corrosion inhibitor. It forms a durable, protective hydrophobic film on metal surfaces, preventing rust in engine coolants, metalworking fluids, and other water-based industrial systems.

A metal part half-submerged in a clear liquid, with the submerged half clean and the exposed half rusty.
While we’ve focused on isononanoic acid as a building block for esters, its utility doesn’t stop there. In its own right, it’s a foundational component for creating some of the most effective corrosion inhibitors on the market. This dual functionality makes it an incredibly efficient tool in a formulator’s arsenal.
The Chemistry of Protection
The magic happens when isononanoic acid is reacted with a base, typically an amine like triethanolamine (TEA), to form an amine salt. This new molecule has a distinct, amphiphilic structure: a polar, water-loving "head" (the carboxylate group) and a long, non-polar, oil-loving "tail" (the C9 hydrocarbon chain). When introduced into a water-based system, the polar head is naturally attracted to and adsorbs onto the metal surface. As countless molecules line up, their non-polar tails orient outwards, forming a tightly packed, hydrophobic (water-repelling) barrier. This microscopic film physically blocks water and oxygen—the key ingredients for rust—from reaching the metal, effectively stopping corrosion in its tracks.
Applications in Practice
This principle is widely used in many industrial fluids. In engine coolants (antifreeze), these isononanoic acid salts are a key part of Organic Acid Technology (OAT) packages, providing long-life protection for all the metals in a cooling system, from cast iron and steel to aluminum and copper alloys. In metalworking fluids, they perform a dual role. Not only do they protect the workpiece and machine tools from rust, but the oily tail also provides a degree of lubricity, reducing friction during cutting and grinding operations. This versatility allows formulators to create simpler, more robust, and longer-lasting fluids that protect valuable equipment.
How Do You Select the Right Polyol to Pair with Isononanoic Acid?
Choosing INA is only half the battle. Pairing it with the wrong polyol can compromise the final lubricant’s properties and cost-effectiveness, failing to meet the specific demands of your target application.
The choice of polyol (e.g., NPG, TMP, PE) determines the final lubricant’s viscosity, thermal stability, and application. Simpler polyols like NPG create lower viscosity fluids, while complex ones like PE create high-viscosity, high-stability lubricants for the most extreme conditions.
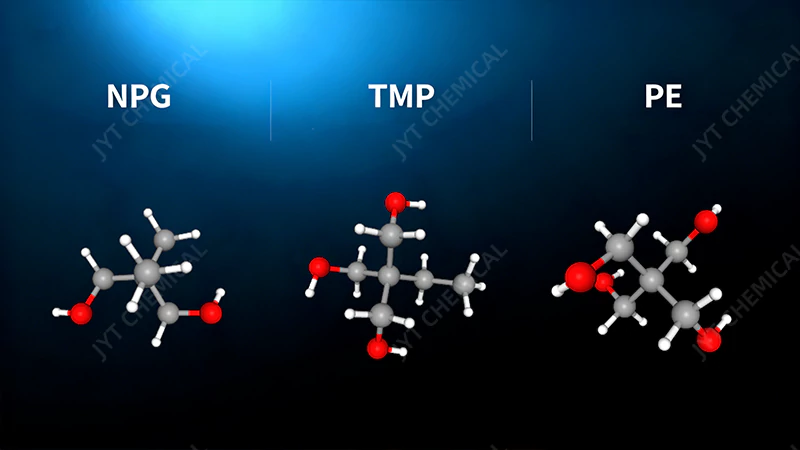
Molecular structures of Neopentyl Glycol (NPG), Trimethylolpropane (TMP), and Pentaerythritol (PE).
Once you’ve chosen isononanoic acid for its stability and low-temperature benefits, the next critical decision is selecting the polyol core. This choice directly dictates the final viscosity and thermal limits of your polyol ester. Let’s look at the common options.
Neopentyl Glycol (NPG)
NPG is a simple diol, meaning it has two reactive sites for acids to attach. Because it forms a relatively small and simple ester, NPG-based POEs have the lowest viscosity. They are ideal for applications where high fluidity is paramount, such as certain hydraulic fluids, low-viscosity refrigeration oils, or as a blending component to reduce the viscosity of a thicker formulation.
Trimethylolpropane (TMP)
TMP is a triol, offering three reactive sites. It’s a versatile workhorse that creates medium-viscosity esters. TMP-based POEs strike an excellent balance between good low-temperature fluidity and strong high-temperature performance. From my experience, they are one of the most common choices for a wide range of industrial and automotive lubricants, including gear oils, compressor oils, and general-purpose synthetic greases.
Pentaerythritol (PE)
PE is a tetraol with four reactive sites, and its derivative, Di-Pentaerythritol (Di-PE), has six. These complex polyols create the highest viscosity and most thermally stable esters. The compact, cross-like structure of PE, combined with multiple ester linkages, results in a molecule that is exceptionally resistant to thermal breakdown. This makes PE and Di-PE the undisputed choice for the most demanding applications on earth, most notably aviation turbine oils.
| Polyol Type | Structure | Resulting Viscosity | Primary Application |
|---|---|---|---|
| Neopentyl Glycol (NPG) | Simple Diol | Low | Low-viscosity fluids, refrigeration |
| Trimethylolpropane (TMP) | Triol | Medium | General industrial & automotive lubricants |
| Pentaerythritol (PE) | Tetraol | High to Very High | Aviation turbine oils, high-temp fluids |
Conclusion
Isononanoic acid is a cornerstone of modern fluid formulation. Its unique branched structure provides the chemical foundation for creating lubricants and corrosion inhibitors with unparalleled thermal stability, low-temperature fluidity, and long-term reliability, empowering formulators to meet the toughest performance challenges.
References
-
- Polyol Ester: Wikipedia, The Free Encyclopedia.
-
- Corrosion Basics: AMPP (Association for Materials Protection and Performance).
-
- Ester Degradation Pathways: Chemistry LibreTexts.