Formulators are under immense pressure. You need to create coatings that are tough and glossy, but also meet tightening environmental regulations. Traditional chemistry often forces a difficult compromise.
Isononanoic acid is a key molecule for modern coatings, enabling the formulation of high-solids, low-VOC alkyd resins with excellent durability. It also serves as a safer, high-performance alternative for creating metal carboxylate paint driers, moving the industry toward greener solutions.

[A robotic arm spraying a glossy, eco-friendly coating onto a car panel]
As formulators, we’re constantly balancing a triangle of demands: performance, cost, and compliance. For years, achieving high performance often meant using high levels of volatile organic compounds (VOCs) or materials with questionable safety profiles. Today, that’s no longer a viable strategy. Consumers and regulators alike are demanding greener products. The good news is that innovative chemistry offers a way out. Isononanoic acid (INA) is one of the most powerful tools I’ve encountered for breaking this compromise, allowing us to build better, safer, and more sustainable coatings from the molecule up.
How Does INA Enable the Shift to Low-VOC Alkyd Resins?
High VOC content in traditional solvent-borne alkyd paints is a major regulatory headache. Simply cutting the solvent often leads to a thick, unworkable resin that compromises the final finish.
Isononanoic acid’s bulky, branched structure acts as a "chain stopper" during polymerization. This reduces the resin’s viscosity, allowing for a much higher solids content. The result is a high-solids alkyd that requires significantly less solvent, directly lowering VOC emissions.
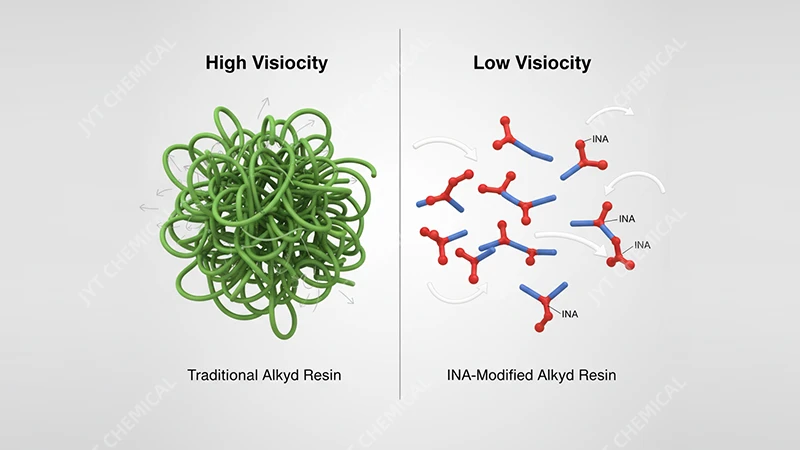
[A diagram showing long polymer chains (high viscosity) vs. shorter, INA-modified chains (low viscosity)]
To understand this innovation, let’s first revisit traditional alkyd resins. They are polyesters formed by reacting a polyol (like glycerol), a polybasic acid (like phthalic anhydride), and a fatty acid derived from oils. The long, linear fatty acid chains are essential for flexibility and air-drying capabilities, but they also cause the polymer chains to become very long and entangled. This entanglement is the primary reason for the high viscosity of conventional alkyd resins, forcing formulators to add large amounts of solvent (VOCs) to make the paint sprayable or brushable. This has been the central challenge for alkyd formulators for decades.
The Role of Isononanoic Acid as a Chain Stopper
Isononanoic acid offers a brilliant solution. As a monofunctional carboxylic acid, it can only react at one end. When introduced into the alkyd formulation, it acts as a chain terminator or "chain stopper." It attaches to the end of a growing polymer chain, preventing it from getting longer. Its unique structure is not only branched but also bulky. This steric hindrance prevents the shorter polymer chains from packing closely together, further contributing to a lower overall viscosity. From my experience in the lab, the difference is dramatic; you can physically see the INA-modified resin flow more easily than its traditional counterpart at the same solids level.
The Path to High-Solids Coatings
This viscosity reduction is the key to formulating high-solids coatings. By using INA, we can pack more resin (the "solids" that form the actual paint film) into the can and use less solvent. This directly addresses the industry’s biggest challenge: reducing VOCs to comply with environmental regulations without sacrificing the performance attributes that make alkyds so popular. These high-solids coatings offer a more sustainable footprint and a less pungent odor, improving the experience for both the applicator and the end-user.
| Alkyd Resin Type | Typical Solids Content | Key Characteristic | Environmental Impact |
|---|---|---|---|
| Traditional Long-Oil Alkyd | 45-60% | High viscosity, requires significant solvent. | High VOC emissions. |
| INA-Modified High-Solids Alkyd | 70-90% | Low viscosity, requires minimal solvent. | Low VOC emissions. |
What is INA’s Role in Creating Durable, High-Gloss Stoving Enamels?
Your baked enamel finish looks great initially, but it yellows, cracks, and loses its gloss when exposed to heat or sunlight. This leads to premature failure and customer complaints.
Isononanoic acid provides excellent thermal and UV stability. When used to create the polyester or alkyd resins for stoving enamels, it improves color retention, prevents yellowing, and enhances chemical resistance, leading to a tougher, more durable, and longer-lasting finish.

[A close-up of a pristine, high-gloss white appliance finish, reflecting light perfectly]
Stoving enamels, also known as baking enamels, are a cornerstone of industrial finishing. They are used on everything from home appliances and office furniture to automotive components. These coatings are cured in an oven, where a chemical reaction (cross-linking) creates an extremely hard and durable film. The quality of that film, however, depends entirely on the quality of the resin backbone. In my experience as a formulator, the biggest long-term failure point for many stoving enamels is the degradation of the resin itself. Traditional fatty acids or other linear acids used in the polyester backbone can contain "weak spots" that are susceptible to oxidation at high temperatures or breakdown from UV radiation. This degradation manifests as yellowing, loss of gloss, and eventually, cracking or chalking of the coating.
Building a More Robust Resin Backbone
Isononanoic acid’s highly stable, saturated branched structure is the perfect defense against this. It contains no double bonds that can easily oxidize, and its carbon skeleton is exceptionally resistant to thermal cleavage. By incorporating INA into the polyester or alkyd resin, you are fundamentally building a more robust polymer. This translates directly into superior performance:
- Excellent Color Retention: The resin resists yellowing even after prolonged exposure to heat during the baking process or from sunlight during its service life. This is especially critical for white and light-colored finishes on appliances.
- Superior Gloss Retention: By preventing the polymer from breaking down, the surface of the coating remains smooth and intact, retaining its initial high gloss for much longer.
- Enhanced Chemical Resistance: The dense, cross-linked film created with stable INA-based resins is less permeable to chemicals, improving its durability against household cleaners, oils, and other common substances.
This principle of building in durability at the molecular level is what separates a premium finish from a standard one. It’s about proactive design rather than reactive protection.
Why is Isononanoic Acid a Greener Choice for Paint Driers?
Traditional paint driers often use acids like 2-Ethylhexanoic acid (2-EHA), which are facing increasing regulatory scrutiny due to health concerns. How do you find a safer, effective alternative without reformulating your entire system?
Isononanoic acid is used to produce metal carboxylate paint driers (e.g., cobalt, zirconium, or manganese isononanoates). It is the industry’s preferred alternative to 2-EHA due to its much more favorable toxicological profile, while still providing excellent drying performance.

[A drop of clear liquid (paint drier) being added to a can of paint, symbolizing a safer additive]
Paint driers are critical additives in any air-drying coating, such as an alkyd-based paint. They are catalysts that dramatically speed up the curing process, which involves the reaction of the paint’s oils with oxygen. Without them, a coat of paint could take days or even weeks to dry. These driers are metal carboxylates—a combination of a metal ion (like cobalt, manganese, or zirconium) and a carboxylic acid. The acid’s job is to act as a carrier, making the metal ion soluble in the oil-based paint binder so it can do its catalytic work.
The Problem with 2-Ethylhexanoic Acid (2-EHA)
For decades, the go-to carrier acid was 2-Ethylhexanoic acid (2-EHA). However, its regulatory status has changed dramatically. In many regions, 2-EHA is now classified as a reproductive toxicant (Category 1B). This has put immense pressure on paint manufacturers to find a safer alternative to protect both their workers and the end-users of their products, forcing a major shift in formulation strategy across the industry.
Isononanoic Acid: The Superior, Safer Solution
This is where isononanoic acid has become a hero for the coatings industry. It serves as a drop-in replacement for 2-EHA in the manufacturing of paint driers, offering several key advantages:
- Favorable Toxicological Profile: Isononanoic acid is not classified as a reproductive toxicant. This makes it a significantly safer and more sustainable choice, helping formulators create products that are compliant with global regulations like REACH and free from hazardous warning labels.
- Excellent Performance: Isononanoates provide solubility and catalytic efficiency that is on par with, or even superior to, their 2-EHA counterparts. The branched structure of INA ensures the metal ions remain well-dispersed and active within the paint binder.
- Low Odor: Driers made with isononanoic acid typically have a lower and less pungent odor compared to those made with other acids, contributing to a more pleasant experience for the painter.
By switching to isononanoate-based driers, formulators can maintain the high performance their customers expect while taking a significant step forward in product safety and environmental responsibility.
How Does INA Improve Coating Weatherability and Chemical Resistance?
Your exterior coating looks great for a year, but then it starts to chalk, fade, and peel. Water ingress and UV radiation are breaking down the film, leading to costly repainting.
The same properties that make INA excellent for lubricants and enamels—hydrolytic and UV stability—also boost a coating’s weatherability. Its structure creates a more water-resistant and UV-stable film, leading to superior long-term durability against sun, rain, and chemicals.

[An outdoor-coated surface showing half faded and cracked, and the other half pristine and new]
A coating’s true value is often measured by how well it stands up to the elements over time. For exterior architectural paints, automotive clear coats, and industrial maintenance coatings, weatherability is the most critical performance metric. This is another area where the fundamental chemistry of isononanoic acid provides a distinct advantage.
Defense Against Water: Hydrolytic Stability
As we discussed in the context of lubricants, the bulky, branched structure of INA provides steric hindrance that protects the ester linkages in a resin from being attacked by water (hydrolysis). In a coating film, this same mechanism helps prevent water from breaking down the polymer binder. This enhanced hydrolytic stability reduces blistering, peeling, and loss of adhesion, especially in humid environments or areas with frequent rain. It creates a more robust barrier that maintains its integrity for longer.
Defense Against the Sun: UV Stability
UV radiation from sunlight is a primary cause of coating degradation. It generates free radicals that attack the polymer backbone, breaking it down. This leads to two common failures: chalking, where the degraded binder turns into a white powder on the surface, and fading, where UV rays bleach the pigments. Because INA’s saturated structure is inherently more resistant to UV breakdown than many traditional fatty acids, resins made with it are less prone to these issues. They maintain their structural integrity, which in turn protects the pigments within the film, leading to better color and gloss retention over many years of outdoor exposure. By building a more UV-stable foundation, you extend the aesthetic and protective life of the entire coating system.
Conclusion
Isononanoic acid is a pivotal ingredient for the modern coatings formulator. It enables the creation of low-VOC alkyd resins, builds superior durability into stoving enamels, and provides a safer, greener foundation for high-performance paint driers, proving that innovation and sustainability can go hand-in-hand.
References
-
- Alkyd Resin: Wikipedia, The Free Encyclopedia.
-
- Stoving Enamel: Corrosionpedia.
-
- Paint Drier (Oil Drying Agent): Wikipedia, The Free Encyclopedia.